High school Design & Technology students often have to be familiar with a range of metal finishes and surface treatments as well as how to prepare a metal surface for finishing. This article summarises this topic and concludes with sample examination questions.
Preparing a metal surface for finishing
Before anything is painted or another finish applied, the surface must be cleaned and free of rust and other debris.
- Use a rag dipped in a solvent such as methylated spirits to clean the surface and remove dirt, grease, and oil (do not breathe in fumes and work in well-ventilated area) or wash with soap to remove dirt
- Use a wire brush or emery cloth (abrasive cloth) to remove dust and oxidation and smooth the surface (wear a face mask to prevent inhalation of particles)
- Dry thoroughly
- Clean, lint-free cloth or rag for wiping
This is necessary so the finish adheres well to the material and the finish is smooth and free of imperfections.
Different types of metal finishing methods
Paint – applied by brush, spray, or dipping to provide color and protect against corrosion.
High temperature paints can be used in heat-vulnerable areas, such as exhaust systems, engines, and stovepipes (these are typically silicone or ceramic-based)
Paint thinners are solvents that can be used to dilute paint, or to clean brushes, or to strip old paint from surfaces.
Ceramic coatings – spray-applied coatings (these are useful for providing heat resistance and corrosion resistance – can handle very high temperatures)
Sealants – protective coatings that fill tiny bumps in surface and create barriers against moisture and contaminants.
Preservatives – chemical treatments (oils, waxes, or solutions) that prevent corrosion
Anodizing – an electrochemical process creating a protective oxide layer on aluminum, can be dyed for color.
Electroplating – a thin metal layer (chrome, nickel, gold, etc.) is applied using electrical current for appearance or protection.
Powder Coating – a dry powder (often a thermosetting or thermoplastic powder) is sprayed on, which clings to all surfaces (so it is good at getting into tiny gaps etc) – then heated to form a durable, uniform coating (thicker, more perfect finish than painting – less likely to chip etc, but requires specialised equipment and curing ovens)
Galvanizing – Coating steel/iron with zinc, typically by hot-dipcoating (where the metal is dipped in molten zinc), for excellent corrosion resistance.
Polishing – Mechanical process using progressively finer abrasives to create a mirror-like reflective surface (many stainless steel items are polished).
Brushing – Creating uniform directional scratch patterns for a satin or matte decorative finish.
Black Oxide – Chemical conversion coating creating a black finish with mild corrosion resistance.
Patina – Chemical solutions creating colored oxide layers, especially on copper, brass, and bronze.
Plastic dip coatings
Offers uniform coverage on complex shapes. Unlike spray coatings, doesn’t lead to overspray / waste. Process:
- Organise a wire or magnetic support to hang item from (so it can hang while drying)
- Use an alcohol solution on a rag to clean surface of grease and debris to ensure plastic bonds well (use wire brush if needed first)
- Shake can of molten plastic dip solution (often a synthetic rubber solution or PVC)
- Some methods require heating the object first
- Insert item into the dipping solution, and slowly life out, ensuring recommended slow dip speed is followed (if you lift it out rapidly, less plastic sticks and it can have runs / splashes / uneven application etc)
- Modern automated machines sometimes heat the plastic after dipping, to improve adhesion and smooth out imperfections
- Hang to dry, ensuring any connecting parts are separated (so plastic doesn’t stick), with something underneath to catch any drips
- Seal dip solution to ensure it doesn’t dry out
- If multiple coats are required, allow sufficient drying time between coats
- Dip again, until desired thickness achieved
- Use an appropriate solvent to clean off any plastic in the wrong place
Safety precautions: Wear chemical-resistant gloves, eye protection, and protective clothing, ensure well ventilated and avoid breathing fumes.
Metal objects can be finished with textured or patterned surfaces (for example, they can be knurled which means they have a textured pattern of ridges or grooves cut into a metal surface (often cross-hatched / diamond pattern or straight ridges) creating a permanent texture that doesn’t wear off easily. Creates improved grip (important for parts that need to be turned or tightened), as well as for aesthetic reasons. Can be applied using a lathe (item spins, while a knurling tool with patterned grooves is held against it, or pressed into softer metals.
Note: Some metals, such as those that acquire a patina, are self-finishing, and do not require a special finishing treatment. Stainless steel is also self-finishing.
Sample examination questions (AS Design & Technology)
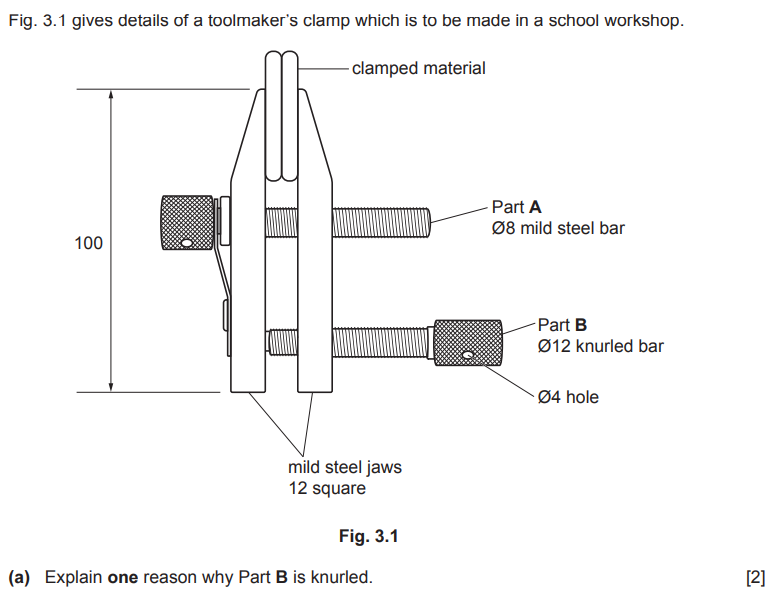

Examiner comment: Most candidates were able to explain why Part B is knurled.
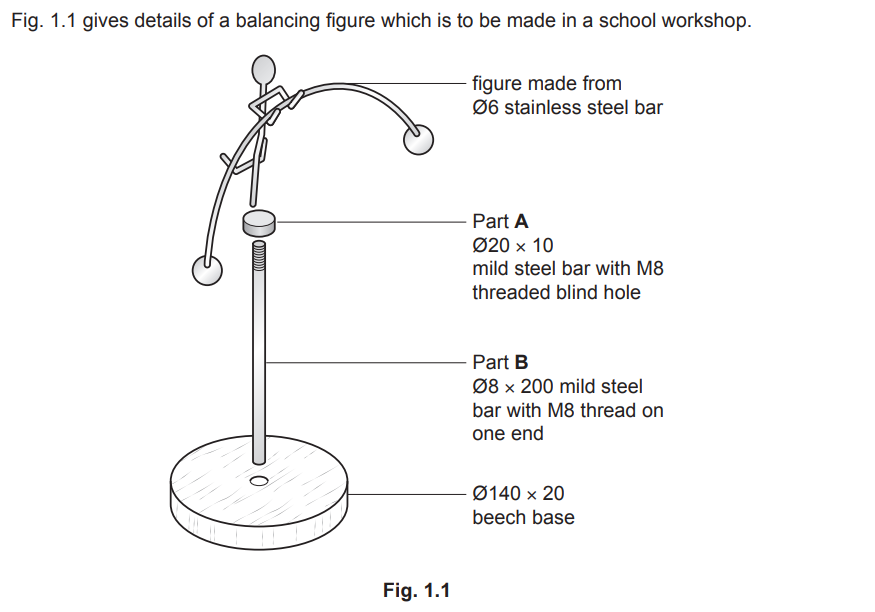



Examiner comment: This was generally answered well with candidates understanding the steps necessary to prepare and apply a finish to the mild steel parts.
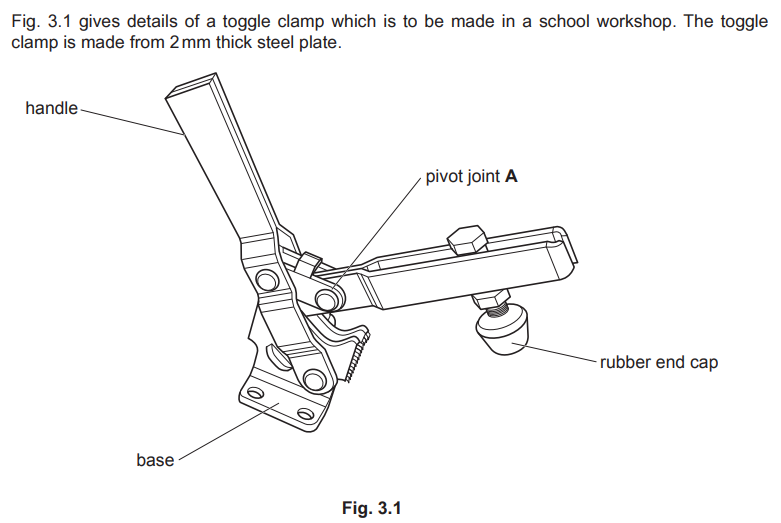


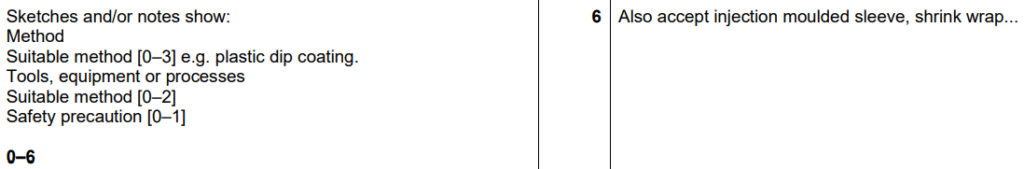
Examiner comment: Candidates found it challenging to explain how a plastic coating could be applied to the handle. However, there were some clear explanations of plastic dip coating. Safety precautions were not always included or were generic.

